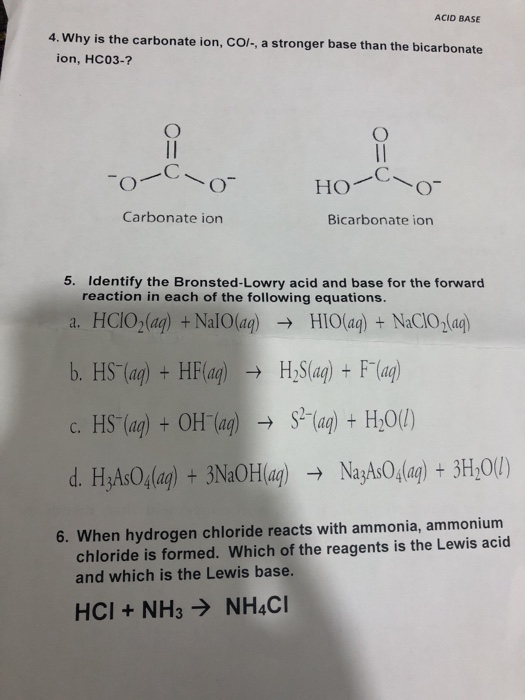
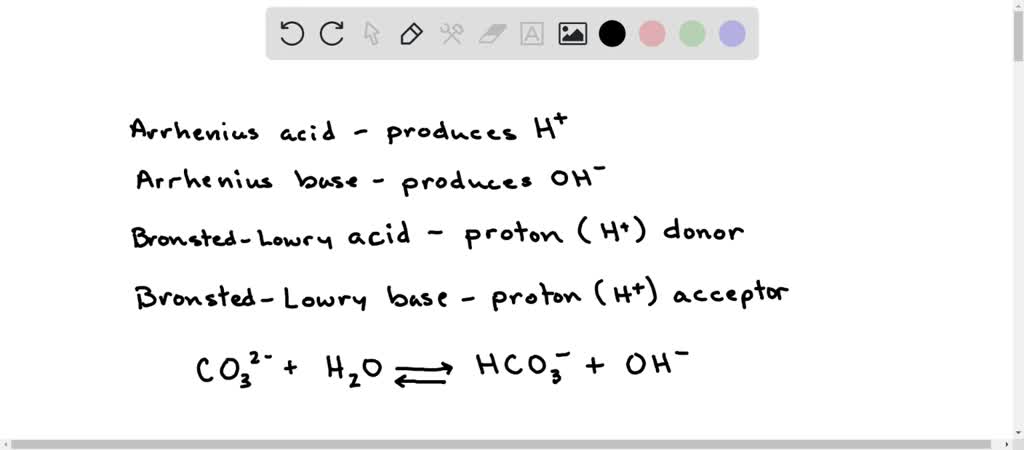
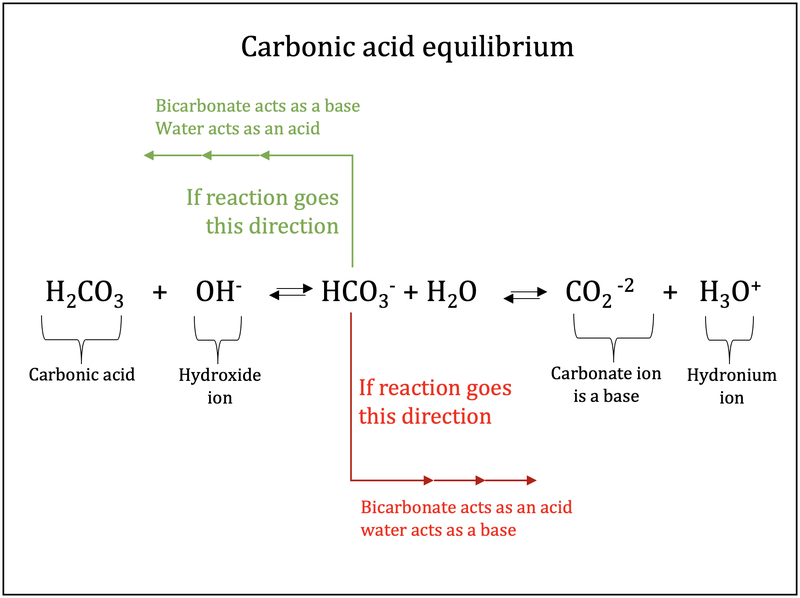
Identify the acid and base which form sodium hydrogen carbonate write chemical equation in support of your answer state whether - Science - Acids Bases and Salts - 13500713 | Meritnation.com

Mechanism of sodium carbonate as a base catalyst for the synthesis of... | Download Scientific Diagram

Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

Reactions. DON'T COPY pink WRITING. Acid + base This is a common reaction and needs to be remembered for exams. ACID + BASE -> SALT + WATER E.g. hydrochloric. - ppt download

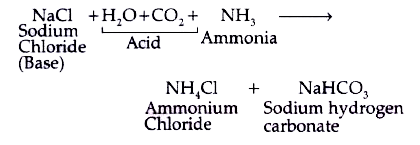


.PNG)